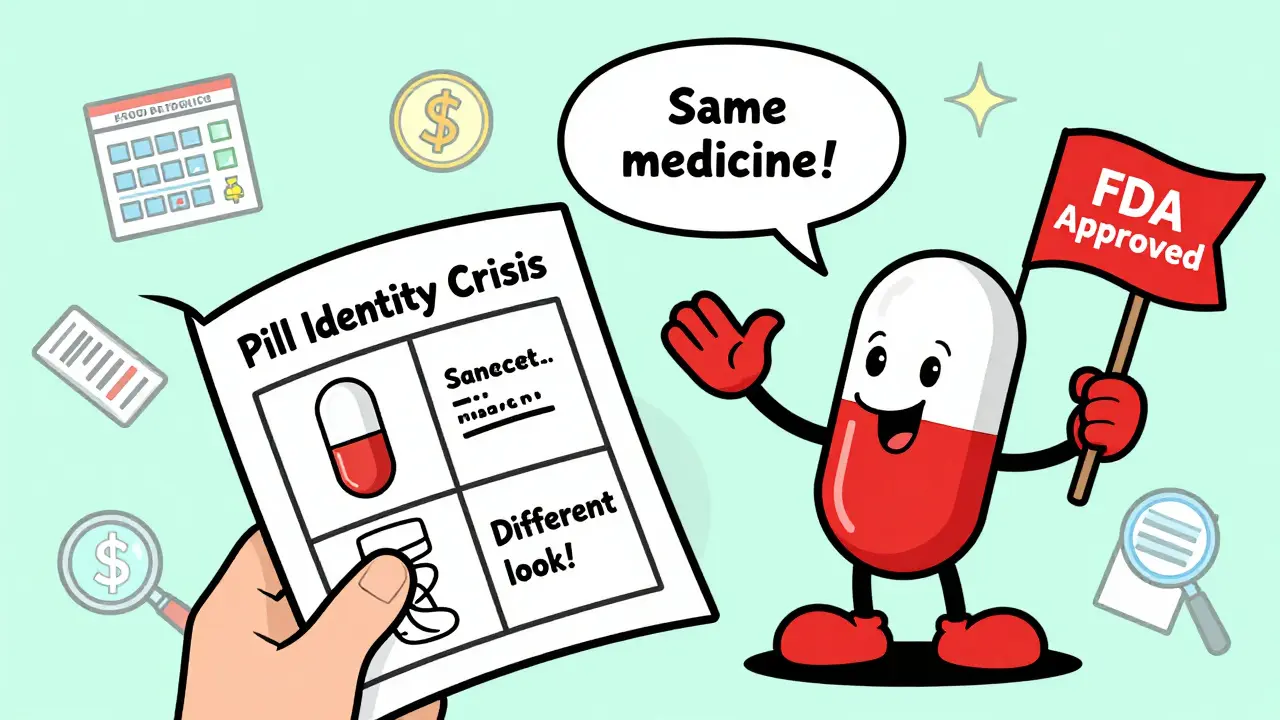
Ever picked up your prescription and thought, “This isn’t the same pill I’ve been taking”? You’re not alone. Many people are confused when their authorized generic looks completely different from the brand-name drug they’ve used for years. Same bottle. Same name on the label. But the pill is a different color, shape, or has a weird marking. What’s going on?
They’re the exact same medicine - inside
An authorized generic is not a copy. It’s the real thing - just without the brand name on the label. The U.S. Food and Drug Administration (FDA) defines it clearly: it’s the exact same drug as the brand-name version, made by the same company, in the same factory, with the same active ingredients and the same inactive ingredients down to the last milligram. There’s no difference in how it works in your body. No difference in effectiveness. No difference in safety. It’s identical.Think of it like buying a soda. The brand-name version has the red label and logo. The authorized generic is the same soda, poured into a plain bottle with no logo. Same taste. Same ingredients. Same fizz. Only the packaging changed.
That’s why authorized generics are often recommended for people who react to inactive ingredients in regular generics - like dyes, fillers, or preservatives. About 4.7% of patients have these kinds of reactions, according to studies in JAMA Internal Medicine. For them, an authorized generic is the safest alternative because it’s chemically identical to the brand-name drug.
Why do they look so different then?
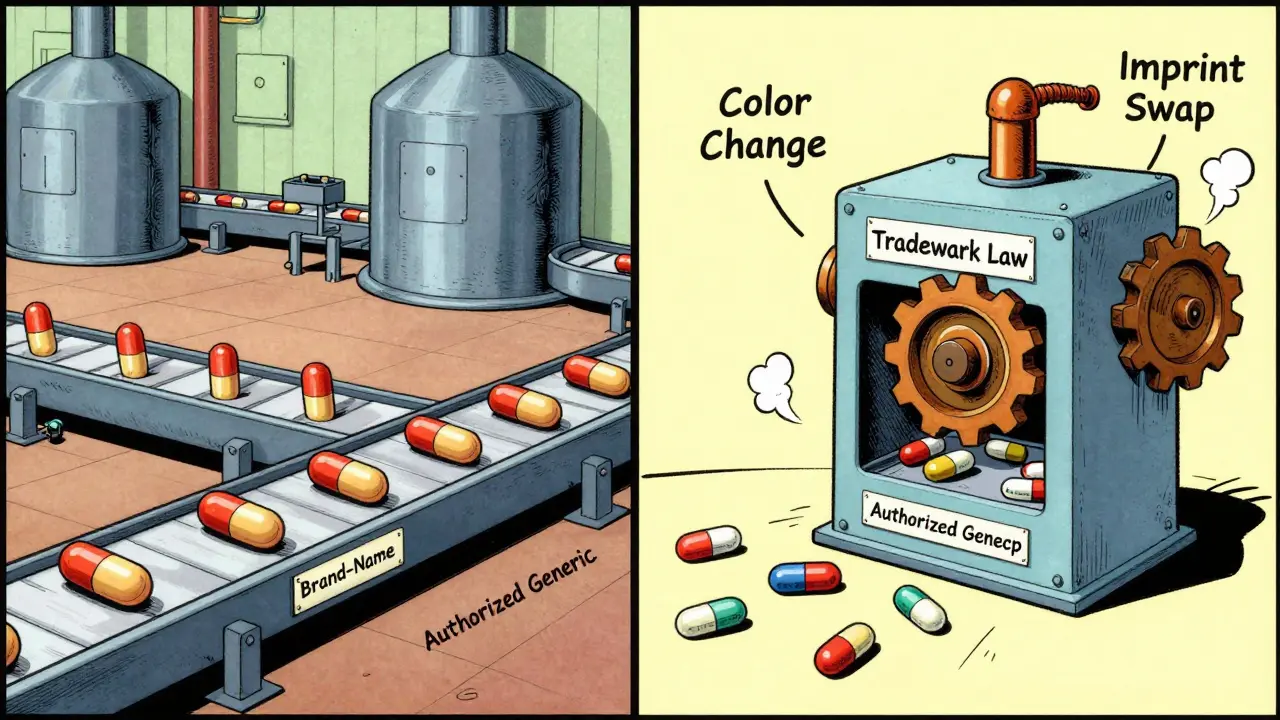
If they’re the same drug, why does the pill look different? The answer isn’t about medicine. It’s about law.U.S. trademark law says no two products can look exactly alike if they’re sold under different names - even if they’re made by the same company. That’s why your brand-name drug has a unique color, shape, and imprint. It’s protected as intellectual property. The FDA requires authorized generics to look different to avoid confusion in pharmacies, hospitals, and at home. This isn’t a loophole - it’s a rule.
According to FDA analysis, 76.4% of authorized generics have a different color than their brand-name counterpart. Nearly 9 out of 10 (89.2%) have a different imprint - the letters or numbers stamped on the pill. The shape and size stay the same in most cases (98.7%), but even small changes in dye concentration - as little as 0.05% - are enough to meet legal requirements without affecting how the drug works.
It’s not random. Manufacturers tweak the coating or dye just enough to satisfy trademark law, but not enough to change how the medicine performs. These changes are carefully documented and approved by the FDA. The goal? Prevent medication errors - like someone accidentally taking two pills because they thought the new one was a different dose.
Authorized generic vs. regular generic: What’s the real difference?
Not all generics are created equal. Regular generics go through a different approval process. They can use different inactive ingredients - binders, fillers, dyes - as long as they’re proven to work the same way. About 64% of regular generics contain at least one inactive ingredient that’s different from the brand-name drug.That’s where authorized generics stand out. They don’t just mimic the brand - they *are* the brand. No substitutions. No swaps. Same recipe. Same manufacturing process. Same quality control.
Here’s a quick comparison:
| Feature | Brand-Name Drug | Authorized Generic | Regular Generic |
|---|---|---|---|
| Active Ingredient | Identical | Identical | Identical |
| Inactive Ingredients | Proprietary formula | Identical to brand | May differ |
| Appearance (color/shape/imprint) | Unique, trademarked | Modified to differ from brand | Often different |
| Manufactured by | Brand company | Brand company or licensed partner | Third-party generic manufacturer |
| Regulatory pathway | New Drug Application (NDA) | Uses brand’s NDA | Abbreviated New Drug Application (ANDA) |
| Typical cost (30-day supply) | $478.23 | $341.05 | $276.17 |
The biggest advantage of an authorized generic? If you’ve had side effects from regular generics - like stomach upset, rashes, or headaches - switching to an authorized generic might solve the problem. Because the inactive ingredients are the same, your body won’t react differently.
Why aren’t all generics authorized generics?
You might wonder: if authorized generics are better, why don’t we see them for every drug?The answer is simple: they’re not always available. Only about 38.4% of brand-name drugs with generic versions also have an authorized generic option. That’s because the brand-name company has to choose to make one. Sometimes they do - to compete with cheaper generics. Sometimes they don’t - because they’d rather keep customers paying full price.
In 18.3% of cases, the authorized generic is priced so close to the brand-name version that there’s almost no savings. That’s frustrating for patients who expect a discount. But even then, if you’re sensitive to inactive ingredients, it might still be worth it.
What patients really say
On Reddit, a thread titled “Authorized generic confusion” had over 140 comments. Most people were startled at first. “I thought I got the wrong medicine,” one user wrote. “Then my pharmacist showed me the FDA page and explained it was the same pill - just different color.”GoodRx reviews show 74.6% of users who switched to an authorized generic rated it positively - mainly because they didn’t have the same side effects they had with regular generics. But 25.4% complained about confusion. “Why does it look different if it’s supposed to be the same?” is the most common question.
Pharmacists say they spend an extra 1.7 minutes per prescription explaining this. That’s not much, but it adds up. That’s why pharmacies like CVS and Walgreens now use visual comparison charts and standardized scripts to help patients understand the difference.
What’s changing in 2025 and beyond
The FDA plans to start listing authorized generics in the Orange Book - the official directory of approved drugs - starting in 2025. Right now, they’re not listed there, which adds to the confusion. Once they are, pharmacists and patients will have a clear, official source to check if a drug is an authorized generic.Some drugmakers are already responding to patient feedback. Pfizer launched an “appearance continuity program” in early 2023, keeping the same pill shape for 12 products and only changing the color in seven of them. The goal? Make it easier for patients to recognize their medication without breaking trademark rules.
Meanwhile, Google searches for “authorized generic vs brand” have jumped 187% since 2020. More people are asking questions. More people are demanding transparency. And that’s pushing the industry to do better.
What you should do
If you’re prescribed an authorized generic and it looks different:- Don’t panic. It’s not a mistake.
- Check the label. It should say “authorized generic” or list the brand name as the reference drug.
- Ask your pharmacist: “Is this the same as my brand-name pill, just without the logo?”
- If you’ve had reactions to regular generics, this might be the better option.
- If the price isn’t lower, ask why - and whether a regular generic would be cheaper and still safe for you.
Remember: appearance doesn’t equal effectiveness. A pill’s color doesn’t change how it works. But your understanding of it can change how you feel about taking it.
Are authorized generics as effective as brand-name drugs?
Yes. Authorized generics contain the exact same active and inactive ingredients as the brand-name drug. They’re made in the same facility using the same process. The FDA considers them therapeutically identical. There is no difference in how they work in your body.
Why do authorized generics cost less than brand-name drugs?
They cost less because they don’t include the marketing, advertising, and brand-building expenses that go into the brand-name version. The manufacturer saves money by using the same production line and skipping the brand packaging. That savings gets passed on to consumers - typically around 28.7% less than the brand-name price.
Can I switch from a brand-name drug to an authorized generic safely?
Yes. Since the ingredients are identical, switching is safe and doesn’t require a new prescription. Many patients switch without even noticing a difference - except for the lower price. If you’ve had reactions to regular generics, an authorized generic is often the safest alternative.
Do authorized generics show up in the FDA’s Orange Book?
Not yet. As of 2025, authorized generics are not listed in the FDA’s Orange Book because they use the brand’s original approval (NDA), not their own generic application (ANDA). Starting in 2025, the FDA plans to include them to improve clarity and reduce confusion.
Why can’t authorized generics look exactly like the brand-name drug?
U.S. trademark law requires that different products - even if identical - have a distinct appearance to prevent confusion. The FDA enforces this rule to avoid medication errors. So, while the medicine inside is the same, the color, imprint, or coating must be changed slightly to comply with legal requirements.
Final thought
The difference in appearance isn’t about quality. It’s about law. It’s about labels. It’s about preventing mistakes in a system that’s built on visual cues. But the medicine? That’s unchanged. The same science. The same safety. The same results.When you see a pill that looks different, don’t assume it’s weaker. Don’t assume it’s fake. Ask. Understand. And know - sometimes, the best version of your medicine is the one that doesn’t wear a brand name.


Comments (13)
innocent massawe
3 Jan, 2026I got my first authorized generic last month and thought I'd been scammed 😅 Turns out it's the same stuff, just no fancy logo. My pharmacist showed me the FDA page and I was like... oh. So simple. Why didn't anyone tell me this before?
veronica guillen giles
5 Jan, 2026Oh wow, so the FDA forces drug companies to make pills look different just so you can't confuse them with... themselves? That's like banning two identical cakes from having the same frosting because they're sold under different names. Brilliant. 🙄
Tiffany Channell
5 Jan, 2026Let me guess - this is just another corporate scam dressed up as patient care. Authorized generics? More like 'brand-name lite' so Big Pharma can keep charging $300 while pretending to be cheap. And don't get me started on how they tweak dye by 0.05% to 'comply' with trademark law. That's not science - that's legal theater.
Ian Detrick
7 Jan, 2026It's fascinating how we've built a medical system where the shape and color of a pill matter more than its chemical composition. We trust science to heal us, but we're terrified of a pill that doesn't look like the one we grew up with. Maybe the real issue isn't the medicine - it's our need for visual identity in everything, even our prescriptions.
Angela Fisher
7 Jan, 2026This is all a lie. I know people who got these 'authorized generics' and ended up in the ER. The FDA doesn't test the *real* stuff - they just approve the paperwork. The dye changes? That's where the toxins hide. They're testing on animals, sure, but humans? We're the lab rats. And they're hiding it behind 'trademark law.' Wake up. This is how they control us.
Neela Sharma
7 Jan, 2026In India we call this 'same juice different bottle' 🌺 My grandma used to say - if the taste is the same, why cry over the wrapper? I switched to authorized generic for my BP med and my body stopped screaming. No rashes. No nausea. Just peace. Sometimes the simplest truth is the one dressed in plain white.
Shruti Badhwar
8 Jan, 2026The data presented here is statistically significant and methodologically sound. However, the absence of longitudinal studies tracking patient adherence post-switch to authorized generics remains a critical gap. Furthermore, the cost differential does not account for potential increases in pharmacy counseling time, which may offset savings at the system level.
Brittany Wallace
9 Jan, 2026I love how this post bridges science and culture. In the U.S. we’re obsessed with branding - even our medicine has to wear a logo. But in other places, like Japan or Ghana, people don’t care what the pill looks like. They care if it works. Maybe we need to stop treating pills like sneakers and start treating them like life-saving tools.
Palesa Makuru
10 Jan, 2026Honestly, I think people who get upset about pill colors are just being dramatic. If you can’t handle a different shade of blue, maybe you’re not ready for adulthood. Also, why are you even taking this drug? Did you even read the label? Or are you just scrolling through Reddit while your body screams for attention?
Hank Pannell
11 Jan, 2026The trademark law angle is actually a brilliant case study in regulatory arbitrage. The FDA leverages IP law to enforce pharmacovigilance without direct regulation - it’s an elegant workaround. But the real kicker? The 0.05% dye variance? That’s below the LOD for most HPLC assays. So technically, it’s undetectable. Which means the entire visual distinction is performative - not pharmacological. The system is designed to reassure, not to inform.
Sarah Little
12 Jan, 2026Authorized generics aren't listed in the Orange Book? That's a compliance nightmare. Pharmacies are using third-party databases that aren't FDA-certified. This is a liability waiting to happen. One misdispense and you're looking at a malpractice suit. Someone needs to fix this.
erica yabut
13 Jan, 2026I can't believe people still fall for this. You think they're the same? Please. The inactive ingredients are changed to make you *think* it's safe. It's a psychological trick. You're being manipulated into thinking you're getting a better deal. Spoiler: you're not. You're just being sold a placebo in a different wrapper.
Tru Vista
14 Jan, 2026So... same pill, diff color. Got it. So why's it cheaper? Oh right. No ads. Makes sense. Also, why do I care? I just want it to work. 🤷♀️